皆さん、アメリカの10歳レベルの理科の教科書をスラスラ読めますか?東大を目指している開成、灘、桜蔭の生徒の皆さんはいかがですか?万一、下記の英文を読んで瞬時に理解できないなら、あなたの英語の実力はアメリカの10歳児より下です。英語の勉強方法が間違っているのです。すぐに「オンライン理系英語塾」のレッスンを受けてください。
What Are Atoms?
Atoms are the building blocks of matter. Unlike blocks that we know, these building blocks are incredibly small. In fact, they are the smallest particles of an element. Atoms still have the same properties as the elements they make up. Elements are also pure substances. This means they are not mixed with anything else. Pure substances such as nickel, hydrogen, and helium make up all kinds of matter. All the atoms of a given element are identical. Atoms of different elements are not physically the same.
Think of something you might have made from LEGOs. You built some shape using the many different sized and shaped blocks. This is much like how atoms combine to become everything we know. If we took only one size and shape of block and put them together, we would make a pure substance. It would be an element. If you take apart anything that you have built, those individual parts are like the atoms. With those small parts, you build bigger things. Sometimes they are all the same type of block. Other times, they may be different kinds of blocks. We use these combinations of different blocks to make more complicated things.
Size of Atoms
Unlike LEGO bricks, atoms are extremely small. The radius of an atom is well under 1 nanometer. That's one-billionth of a meter. Such a number is hard to imagine. Consider this: trillions of atoms would fit inside the period at the end of this sentence. In other words, atoms are way too small to be seen with the naked eye.
Subatomic Particles
Although atoms are very tiny, they consist of even smaller particles. Atoms are made of protons, neutrons, and electrons:
- Protons have a positive charge.
- Electrons have a negative charge.
- Neutrons are neutral in charge.
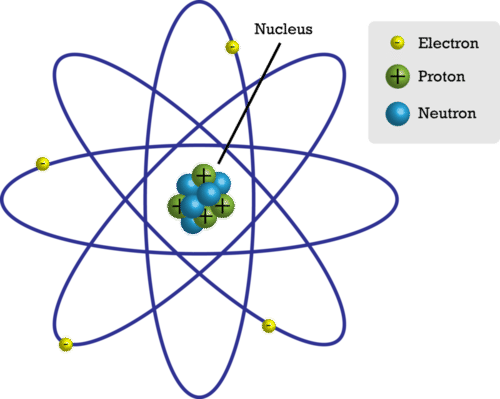
Parts of the Atom
Figure below represents a simple model of an atom. Models help scientists make sense of things. Perhaps they are either too big or too small. Maybe they are just too complicated to make sense of. This simple model helps scientists think about the atom. Is this how the atom really looks? Not exactly! Remember, a model helps us make sense of things. They may not be an exact copy of the object. You will learn about more complex models of atoms in the coming years, but this model is a good place to start.