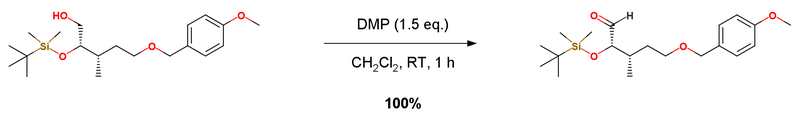
Dess-Martin Oxidation - 01
Dess-Martin periodinane (1.52 g, 3.6 mmol) was added to a stirred solution of alcohol (2b) (660 mg of approximately 90% purity, 1.7 mmol) in dichloromethane (25 mL) under an atmosphere of nitrogen. The mixture was stirred for 1.25 hours then diluted with dichloromethane (50 mL). The organic phase was washed with a mixture of saturated aqueous sodium bicarbonate and 0.25 M sodium thiosulphate solution (1 : 1), then saturated aqueous sodium bicarbonate then brine, then dried (Na2SO4), filtered and reduced in vacuo. Flash chromatography over silica, eluting with ethyl acetate : heptane mixtures 10 : 90 to 40 : 60 gave ketone (2c) (611 mg, quantitative) as a white solid.
6-butyl-3-(3,3-dimethyl-2-oxobutyl)-2-ethyl-5-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}pyrimidin-4(3H)-one 6-Butyl-2-ethyl-3-(2-hydroxy-3,3-dimethylbutyl)-5-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}pyrimidin-4(3H)-one (2.9 g) was dissolved in dichloromethane (60 mL), 1,1,1-tris(acetyloxy)-1,1-dihydro-1,2-benziodoxol-3(1H)-one (2.8 g) was added, and the mixture was stirred for 1 hr. A saturated aqueous sodium hydrogen carbonate solution and sodium thiosulfate hydrate were added, and the mixture was further stirred for 1 hr. The reaction mixture was extracted with ethyl acetate, washed with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography to give the title compound (2.9 g, 100%) as a solid. 6-butyl-3-(3,3-dimethyl-2-oxobutyl)-2-ethyl-5-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}pyrimidin-4(3H)-one, yield 2.9 g, 100%.
To a stirred solution of 5.25 g of Dess-Martin-periodinane 9 (18.8 mmol, 1.5 equiv) in 28.2 mL of dichloromethane at rt was added a solution of 4.61 g of primary alcohol 14 (12.5 mmol, 1 equiv) in 43 mL of dichloromethane and the resulting solution was stirred at rt for 1 h. Diethyl ether (62 mL) was added followed by the addition of 62 mL of saturated aqueous sodium bicarbonate containing 15.6 g of sodium thiosulfate. This solution was stirred at rt for 5 min, diethyl ether (62 mL) was added and the layers were separated. The organic layer was washed with 62 mL of saturated aqueous sodium bicarbonate and 62 mL of water. The organic layer was then dried over magnesium sulfate, filtered and solvents were removed in vacuo. Purification by column chromatography on silica gel (15 % diethyl ether-petroleum ether) gave 4.59 g (quantitative) of a clear colourless oil.
To a solution of the alcohol S7 (180 mg, 0.70 mmol, 1 eq) in CH2Cl2 (5 mL) under argon at 0 ℃ was added Dess-Martin periodinane (358 mg, 0.84 mmol, 1.2 eq). The reaction was stirred 1 h at RT and cooled at 0 ℃ before addition of petrol ether (5 mL). The white suspension is then filtered through a pad of silica (petrol ether/Et2O 1/1 (200 mL)). The volatiles were removed under vacuo to give the expected aldehyde 16 as a yellow oil (180 mg, quant) clean enough to proceed the next step.
To a solution of alcohol 13' (58 mg, 0.261 mmol) in 5 mL DCM was added DMP (144 mg, 0.340 mmol). The mixture was stirred at room temperature for 1 h then diluted with 5 mL of DCM and washed with 3 mL of saturated aqueous Na2SO3 solution and 3 mL of saturated aqueous NaHCO3 solution. The organic phase was dried over anhydrous MgSO4, filtered and concentrated at reduced pressure. Chromatography of the residue (hexane/EA = 3:1 to 1:1) afforded the desired product 6 (58 mg, quant.) as a colorless oil. product 6, yield (58 mg, quant.).
デス・マーチン酸化 Dess-Martin Oxidation
・後処理は、NaHCO3/Na2S2O3 水溶液を加えて、クエンチすることが多い。
もしくは、反応溶媒を除去して、そのままカラム精製する。
・1等量の水を添加すると、反応が加速されることが知られている。(10.1021/jo00103a067)
・DMP 自体が市販され、容易に入手可能になったので、
酸化反応のファーストチョイスとして使われることが増えた。
↓応援クリックしてくれると励みになります!