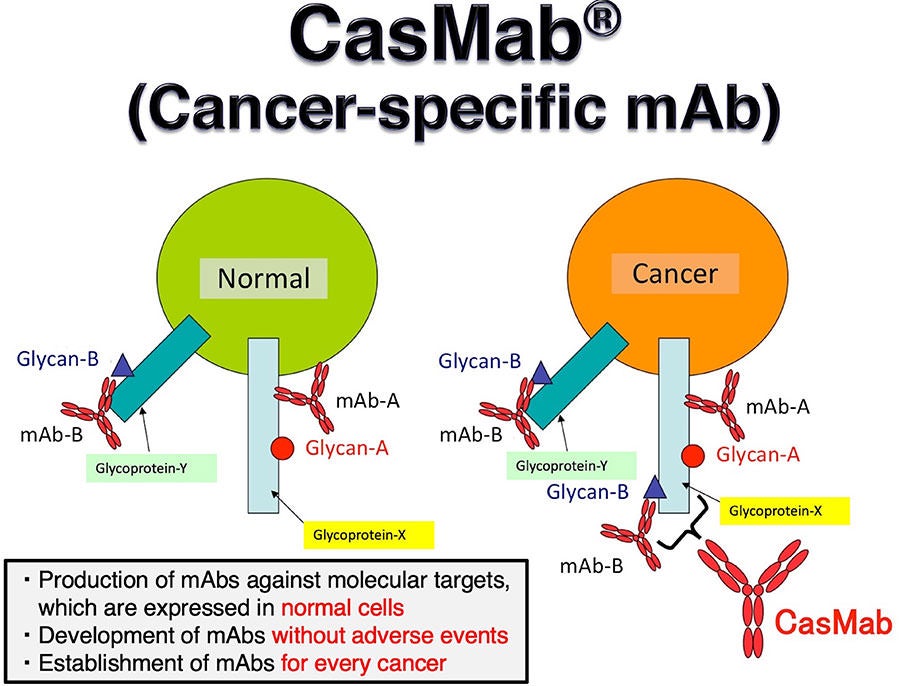
HER2に対するがん特異的抗体(H2CasMab-2/H2Mab-250)を導入したCART細胞(FT825/ONO-8250)の第I相臨床試験にて、第一例目の患者に投与
FT825 / ONO-8250 iPSC-derived CAR T-cell Program
- First Patient Treated in Phase 1 Study with HER2-targeted CAR T-cell for Advanced Solid Tumors. Under its collaboration with Ono Pharmaceutical Co., Ltd. (Ono), the Company is conducting a multi-center, Phase 1 study to assess the safety, pharmacokinetics, and activity of FT825 / ONO-8250 as monotherapy and in combination with monoclonal antibody therapy in patients with advanced solid tumors (NCT06241456). The first patient was diagnosed with HER2-positive gastroesophageal junction (GEJ) adenocarcinoma, had progressed after receiving multiple lines of treatment including HER2-targeted therapies, and was administered standard conditioning chemotherapy followed by a single dose of FT825 / ONO-8250 as monotherapy at 100 million cells. Designed using the Company’s iPSC product platform, FT825 / ONO-8250 incorporates seven synthetic controls of cell function including a novel cancer-specific H2CasMab-2 CAR, which has exhibited similar potency with greater specificity for cancer cells expressing HER2 compared to trastuzumab in preclinical studies.
https://fatetherapeutics.com/wp-content/uploads/2023/11/SITC-2023-FT825-ONO8250-Poster-Final.pdf
https://www.liebertpub.com/doi/10.1089/mab.2023.0033
https://www.sciencedirect.com/science/article/abs/pii/S0969212624000467?dgcid=coauthor