In lithium ion battery anode materials, natural graphite and artificial graphite has always occupied the vast proportion, but as people for the demand of battery energy density gradually increased, graphite capacity theoretical limit of 372 mah/g will no longer meet the demand, and graphite anode structure defects limited as lithium ion battery anode material cycle stability and charge and discharge efficiency, and hard carbon has isotropic structure characteristics, greater layer spacing, charge and discharge when lithium ion diffusion speed and has good multiplier performance, make hard carbon in a good application in the field of lithium ion batteries.
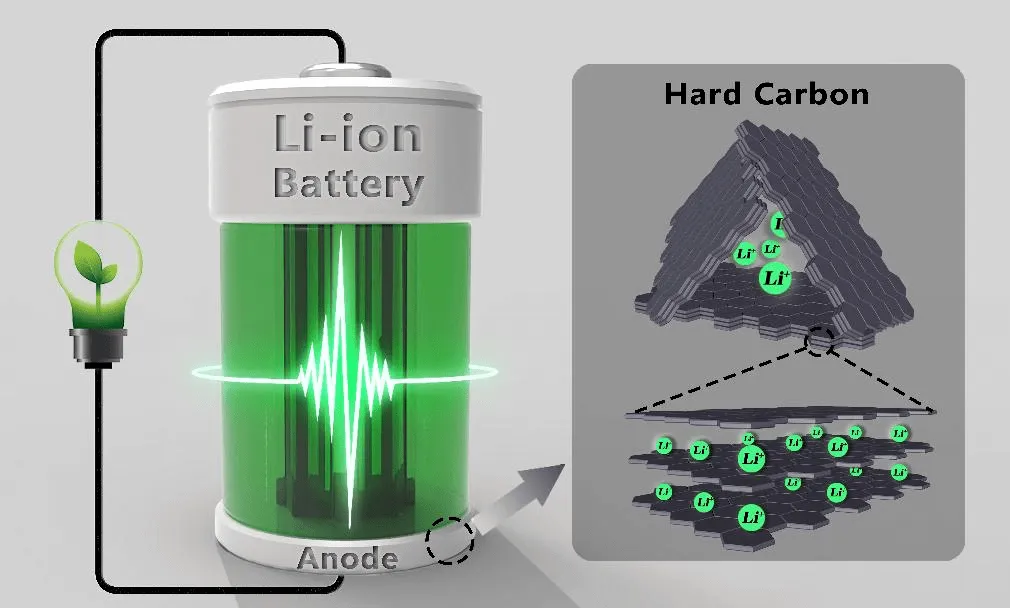
At present, due to the tight supply of lithium resources, the development of lithium battery industry is restricted to some extent, and it has become an urgent need to find lithium battery replacement technology. Sodium-ion batteries, which have similar working principles with lithium-ion batteries, have been valued, and their industrialization has also been put on the agenda. The outstanding advantage of sodium ion battery is more reflected in the cost of raw materials. Sodium ranks sixth in the crust, and is abundant in scarce lithium resources.
Hard carbon is more apart than the graphite layer and can form a thermal stable intercalation compound with sodium, compared with the soft carbon sodium storage capacity, so it is widely considered as the most promising cathode material for emerging sodium ion batteries.

1.What is hard carbon
Hard carbon refers to the difficult graphite carbon, is a kind of pyrolysis carbon obtained by pyrolysis of polymer, petrochemical products, biomass, etc. The large number of heteroatoms such as H, O, and N in the precursor hinder the formation of the crystallization region during heat treatment, leading to difficult graphitization at high temperatures above 2500℃.
In the process of pyrolysis, the carbon layer has a tendency to grow in plane, but the cross-linking structure in macromolecules hinders its plane growth. Therefore, the carbon layer in hard carbon can not extend indefinitely to a graphite-like sheet structure, only the carbon layer stacking structure can appear in the short range, and is disordered in the long range. The hard carbon structure is dominated by amorphous parts.
Due to the disordered accumulation of some carbon layers, defects and holes appear, while the other part of the carbon layer has a graphite microcrystal structure, which are not oriented and cross-linked to each other. Some researchers believe that the molecular weight of the precursor has an impact on the microstructure of hard carbon formed after pyrolysis. With the increase of molecular weight, the graphitization degree of hard carbon gradually becomes higher, and the specific surface area gradually increases.
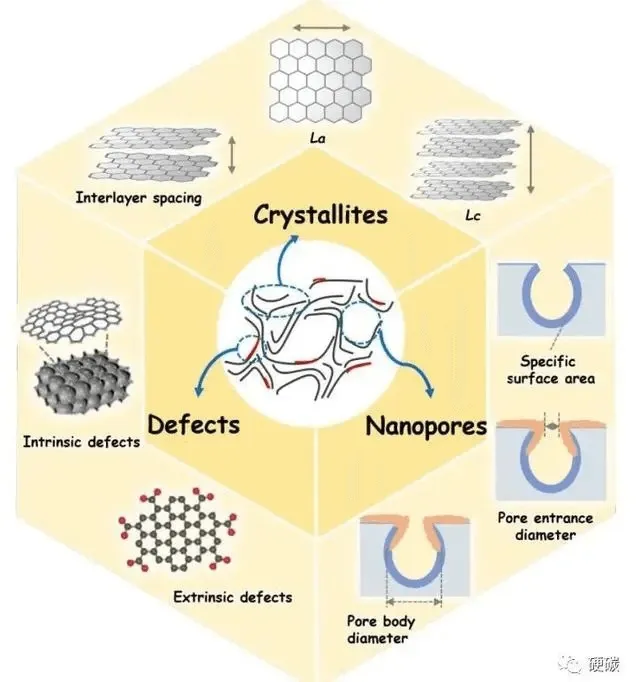
2.Advantages of hard-carbon materials
Hard carbon is conducive to the embedding of lithium without causing significant expansion of the structure, and has good charge and discharge cycle performance. Compared with the graphite layer, the hard carbon has a large spacing and many micropores, corresponding to the ground lithium ion embedded and detached lithium storage active sites, which has a larger specific capacity.
Moreover, hard carbon and PC electrolyte compatibility is better, more suitable for working at low temperature. In addition, hard carbon also has the advantages of large rate of charge and discharge performance and long cycle life.
The molar mass of sodium ion is 3 times that of lithium ion, and the diameter is 1.3 times that of lithium ion, which leads to the sodium ion cannot be reversible embedded and detached among the graphite layers in the effective potential window. At the same time, the compounds embedded in sodium ion-graphite are not thermodynamically stable and easy to form a large number of "dead sodium".
Compared to graphite, such as material, hard carbon material cannot graphitization and carbon layer alignment regularity than soft carbon, formed between more pores and convenient sodium embedded and stripping, and hard carbon has high sodium storage capacity, low sodium storage voltage, cycle stability, is the current preferred sodium ion battery anode material.
Although the special structure of hard carbon makes it have high capacity and good multiplier performance, there are also some disadvantages, such as low couefficiency in the first week, low vibration density and severe voltage polarization.
3. hard carbon precursor and classification
Depending on the carbide temperature of pyrolysis, hard carbon materials can be divided into high temperature between 1000-1400℃ and 500-1000℃. According to the different carbon sources, it can be divided into resin carbon (such as phenolic resin, epoxy resin, polybran alcohol resin, etc.), organic polymer carbon (such as PVA, PVC, PVDF, PAN, etc.), carbon black (acetylene black prepared by CVD method, etc.), biomass carbon (such as plant residues and shell), etc.
The precursors of hard carbon preparation include asphalt, biomass, sugar, phenolic resin, organic polymer, and so on. The hard carbon materials prepared by different substances show similar charge and discharge curves.
① asphalt precursor
As a low-cost petrochemical by-product, asphalt is a better hard carbon precursor due to its high carbon content, wide raw material sources and low price. However, the preparation of hard carbon from asphalt requires pretreatment, because the asphalt is easy to graphitize to form a graphite-like structure in the process of carbonization. Moreover, the asphalt base is easy to form an orderly structure in the process of high temperature cracking, so its storage capacity is very low, less than 100 mAh/g.

② Biomass precursor
Biomass has a wide range of green sources, and itself has rich heteroatoms and a unique microstructure, which can be used as a precursor of hard carbon. Some researchers use grapefruit peel to prepare hard carbon material for carbon source. They concluded that the excellent performance of the samples is closely related to the unique pore structure of the material. This structure helps the material to be in full contact with the electrolyte, providing channels and more lithium-embedded sites for Li+ transport inside the material.
Some researchers also used the groundnut shell as the carbon source to prepare the hard carbon material. After research, they believed that under the same experimental conditions, the rich pore structure of the material could help to improve the lithium-embedded capacity and the cycle stability of the material. Some researchers used corn straw as carbon source to prepare carbon material with porous structure, and showed excellent ratio and circulation performance.
They believe that the existence of micropores makes the material have a large number of defect sites, providing an active site for the storage of lithium ions and improving the lithium storage capacity, while the existence of mesopres and large pores shortens the transport distance of lithium ions and improves the migration rate of lithium ions. But biomass from nature often contains some impurities that need to be removed before being applied to batteries. And despite the sustainability and richness of biomass, the overall cost is still higher than that of graphite and soft carbon.
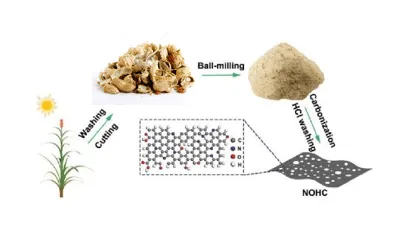
③ Organic polymer precursor
Compared with biomass, the molecular structure of organic polymer is relatively simple and controllable, and the relevant molecular structure can be designed according to the need. It is an excellent precursor for the preparation of hard carbon. Some researchers use phenolic resin as the carbon precursor, obtain resin-based hard carbon material, and use it as the electrode material for lithium ion battery anode material and supercapacitors. The lithium ion battery capacity can reach 526 mAh/g, and the first coulomb efficiency can reach 80%.
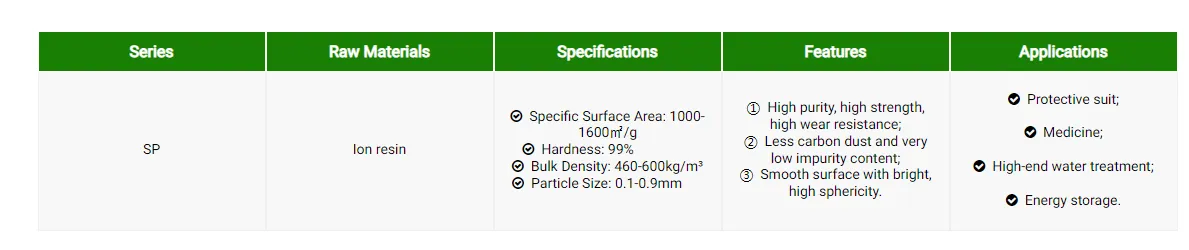
Ion Resin Based Activated Carbon

Ion resin based activated carbon is the core product of renewable raw material activated carbon. It is the latest generation of spherical activated carbon processed with special ion resin as raw material and using the world's leading spherical activated carbon production technology. It is widely used in high-end water treatment, pharmaceutical industry, military and civil protection products, electrode energy storage and other fields.
They believe that the higher porosity is one of the reasons for the higher lithium-embedded capacity. The structure of hard carbon mainly changes with the carbonization temperature, and the layer spacing and disorder decrease with the temperature increases, which affects the sodium storage properties of the material. And by regulating the formation of hard carbon pore structure is beneficial to improve the electrochemical properties.
A researcher Kamiyama team found that with the increase of heat treatment temperature, the interlayer distance decreases, the specific surface area decreases, and the internal pore increases. The unique macroporous phenolic resin increases the total volume of the inner pore, thereby increasing the sodium ion capacity. However, the production cost of organic polymer is relatively high, and the large-scale preparation is still difficult.