When acquiring a nutritional supplement, it is vital that the company has a high quality standard that follows the highest possible standards. There are a number of requirements to look for, including CGMP, ANSI, NSF, and also CFSAN. Each has details needs that a firm should meet to ensure that their item is secure.
CGMP
The CGMP quality standard of nutritional supplements is an important element of nutritional supplements. The standards must be followed in order to be identified as safe and also reliable for consumers. The FDA imposes these criteria by examining all celebrations along the chain of guardianship. Although it does not issue qualifications, the FDA will provide warning letters to parties that stop working to abide by the standard. Additionally, supplement makers can select to hire independent organizations to perform audits as well as give GMP accreditations.
To satisfy the CGMP high quality criterion, makers have to first test new raw components to make certain that they are cGMP-compliant. Makers are likewise called for to examine each great deal of active item ingredients. This testing procedure starts by establishing identity requirements. Non-active ingredients need to likewise be defined for pureness, composition, and toughness.
Great manufacturing techniques are crucial to nutritional supplement high quality. The FDA calls for supplement suppliers to follow strict standards in their production processes. This makes sure that items are secure and also devoid of impurities. In addition, it requires manufacturers to maintain great hygiene techniques as well as clean their centers on a regular basis. This will ensure secure handling, storage space, and also monitoring.
ANSI
The American National Standards Institute (ANSI) is a personal not-for-profit organization that develops as well as collaborates voluntary requirements for the United States. These criteria are based on participation and also due-process principles. The NSF/ANSI quality requirement for dietary supplements was created by the NSF Joint Board on Dietary Supplements with input from the regulatory neighborhood. Its usage by the nutritional supplement industry assists ensure that supplements meet governing demands.
The USP is responsible for setting public criteria for pharmaceuticals, nutritional supplements, and food components. The organization works together with services, academic organizations, and also governments to create and also maintain these criteria. Their scientists collect data as well as execute lab tests as well as generate reports that are reviewed and also approved by a skilled board. These criteria are published in the form of item essays and compendium.
The NSF/ANSI top quality requirement for dietary supplements is established by a committee comprised of public health and wellness authorities, consumer rate of interests, and supplement industry representatives. The criterion is openly available, as well as lots of sellers such as CVS and also Amazon will accept products licensed to this requirement.
NSF
Customers can have peace of mind when it comes to dietary supplements by picking products that are certified by NSF International. Qualified products go through comprehensive screening to fulfill strict top quality criteria. They likewise bring the NSF seal. The NSF mark on a product provides consumers comfort, as well as it ensures that the label will certainly match the supplement truths panel.
This certification program secures customers from taking in infected nutritional supplements. It inspects products for 280 prohibited compounds and undeclared components. It additionally verifies that the product consists of only the active ingredients provided on the tag. The NSF criterion is identified by the NFL, MLB, PGA, as well as the Canadian Facility for Principles in Sports.
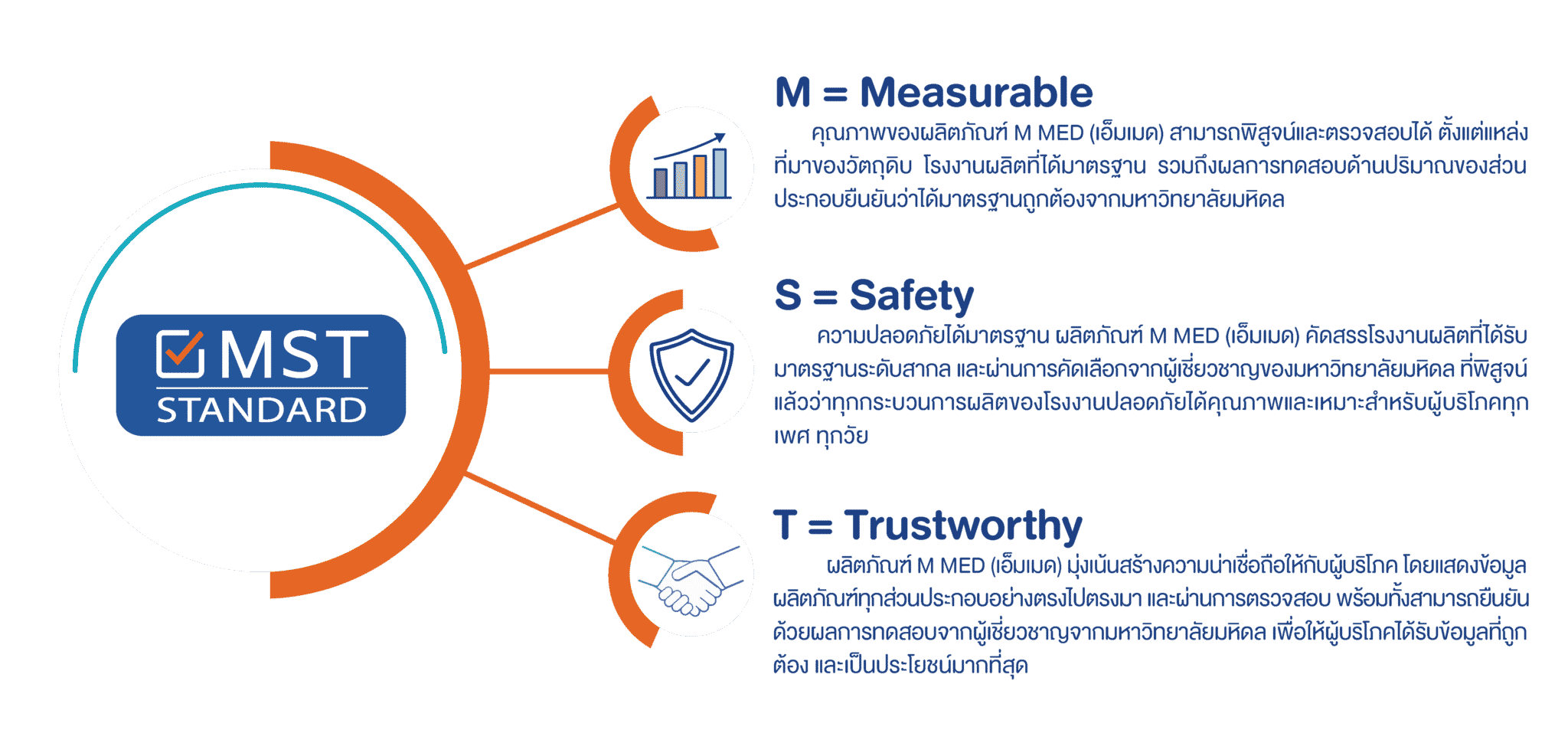
The NSF MST STANDARD is the new high quality standard for dietary supplements established by GRMA. This American National Criterion for dietary supplements has actually been revised by GRMA and other leading health organizations. The NSF MST STANDARD outlines the quality requirements for dietary supplements as well as provides guidelines for producers.
CFSAN
The present excellent manufacturing technique (CGMP) for nutritional supplements is called for by the U.S. Food and Drug Administration (FDA). Under this rule, dietary supplement makers have to follow specific needs for the manufacturing, product packaging, as well as labeling of dietary supplements. Additionally, manufacturers must follow great production methods (GMP) for food.
NSF International carries out audits of nutritional supplement makers twice per year to guarantee conformity with the standard and also full implementation of rehabilitative activities. NSF grants the "GMP Registered" mark to business that fulfill their requirements for manufacturing. NSF also carries out on-site facility audits to ensure manufacturers are following the stringent standards.
The CFSAN MST criterion quality standards for dietary supplements are very important to customers. These standards need that producers carry out identification testing of inbound raw materials and finished products. They likewise call for proper cleansing of tools as well as appropriate training of staff. Manufacturers must also execute in-process controls to guarantee uniformity in product high quality. The company should send records of major negative events to the FDA within 14 days of the event. On top of that, suppliers must keep records of non-serious adverse events.
Import signals
A supplier of dietary supplements requested that the FDA problem import notifies pertaining to beta-alanine originated from Chinese plants. Due to the fact that beta-alanine does not have a National Drug Recognition Number (NDIN), the item is potentially dangerous. While the FDA has not assessed any information on the security of this active ingredient, it has found proof that it might be weakened.
An import alert is a kind of warning that is issued when the FDA locates that an ingredient is illegal or uncertified. While it does not include the Department of Justice, it does call for considerable evaluation and also clearance be issued. For example, if a company declines FDA foreign examinations, it may be detained for a time period.
Voluntary actions
The Volunteer Activities in MST criterion quality criterion of the dietary supplements sector were developed to aid make certain that dietary supplements fulfill quality and also safety and security needs. In other words, items that fall short to meet the Discover more MST criterion's demands are taken into consideration adulterated. This holds true also if these products are devoid of defects.
The USP creates requirements for nutritional supplements, medicines, and also food components. These criteria are recognized by the FDA and also are enforceable. The firms that make these items need to fulfill these standards. On top of that, the USP has established volunteer actions for the MST STANDARD's quality standard.
The USPC criteria are public standards. Prevalent use of the USPC criteria can aid ensure the top quality of nutritional supplements. It likewise could help save FDA sources by getting rid of the requirement for repeated validation and review of items. Nevertheless, this approach might cause safety gaps.
Import examinations
Import assessments of MST STANDard top quality criterion of dietary supplements are called for by the US Food and Drug Administration. Throughout these examinations, the FDA personnel will examine nutritional supplement manufacturing facilities for conformity with the top quality requirement. These assessments might cover the entire CGMP needs or a minimal number of products. Each examination needs to consist of at the very least one finished nutritional supplement item, but additional products might be covered if considerable shortages are determined. Identifying demands may likewise be addressed throughout the evaluation, including the Supplement Information label, negative occasion reporting, irritant labeling, and also other appropriate labeling requirements
Import assessments of dietary supplements may include a physical exam, label examination, as well as example collection. These procedures are detailed in the FD&C Act 413(a) needs.
Identifying demands.
Labeling requirements for dietary supplements ought to follow the demands laid out in the Dietary Supplement and Nonprescription Medicine Customer Protection Act (DSNPDCA). A label must include the name as well as address of the manufacturer and the date of manufacture. The name and address need to project and also quickly legible.
A dietary supplement may consist of a range of ingredients, including a vitamin, mineral, amino acid, enzyme, or mix of active ingredients. These items must be accepted by the food authorities and meet an assisting minimum value for every nutrient. Some nutrients, such as propionic bacteria cultures, are not accepted as food supplements. These products might be drugs in the Danish Medicines Firm (DMA) however should not be marketed as food supplements up until they are registered. Nutritional supplements need to additionally have efficiency as well as safety and security data. Moreover, the items have to not make health and wellness cases or advertise disease treatment. Finally, they have to likewise carry compulsory cautions as well as consist of special labeling for the product's components.
A label for dietary supplements must include the net quantity of materials (NPV), which defines the quantity of supplement in the container. This value can be expressed as a weight, a procedure, or both. The product should be classified in either the metric system or the United States popular system.